Brand-Name Drugs: What They Are, Why They Cost More, and When They Really Matter
When you hear brand-name drugs, the original versions of medications developed and marketed by pharmaceutical companies under a proprietary name. Also known as originator drugs, they’re the first to hit the market after years of research and clinical trials. These are the pills you see advertised on TV, prescribed by your doctor, and often the first option your pharmacy offers—until they’re not. Most people don’t realize that behind every brand-name drug is a legal shield called a patent protection, a 20-year legal monopoly granted to the drug developer to recoup R&D costs. Once that expires, other companies can make identical versions called generics. But here’s the catch: not all brand-name drugs are replaced by generics overnight, and sometimes, you shouldn’t switch at all.
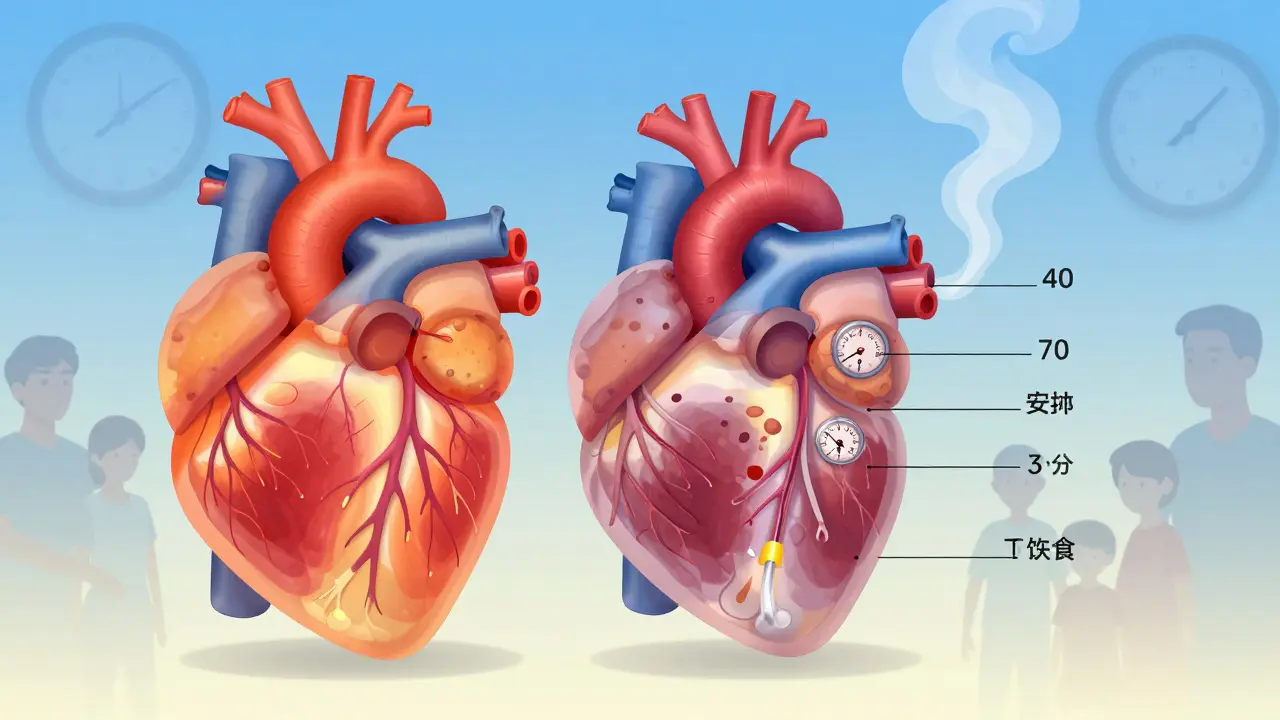
Why do brand-name drugs cost so much more? It’s not just marketing. The price includes the cost of failed drugs, clinical trials that took a decade, and the risk of investing billions with no guarantee of success. But once the patent expires, generic manufacturers don’t have to repeat those expensive tests—they just prove their version works the same. The FDA approval, the official U.S. government process that ensures drugs are safe, effective, and meet quality standards for generics is strict, but it’s faster and cheaper. That’s why generics can cost 80% less. Still, some patients report differences in how they feel on generics versus brand-name versions. That’s not always placebo. For drugs with a narrow therapeutic index—like warfarin, thyroid meds, or seizure drugs—even tiny variations in absorption can matter. That’s why switching back to a brand-name drug is sometimes medically necessary, not just a luxury.
And it’s not just about cost or chemistry. The system around brand-name drugs affects everything: how insurers negotiate prices, how pharmacists recommend alternatives, and even how often you refill your prescription. When a drug is still under patent, there’s no competition—so prices stay high. Once generics enter, prices drop fast. But sometimes, the brand-name company finds ways to delay generics, like tweaking the formula slightly or filing legal challenges. That’s where Paragraph IV certifications, a legal tool used by generic drug makers to challenge patents before launch come in. These filings can speed up access to cheaper versions, but they also trigger lawsuits that can delay savings for years.
So when should you stick with a brand-name drug? If your doctor says so. If you’ve had a bad reaction to a generic. If your condition is sensitive to tiny dose changes. If your insurance won’t cover the generic, or if you’re part of a clinical trial. But if you’re stable, healthy, and paying out of pocket? Generics are almost always the smarter choice. The brand-name drugs you see on the shelf today are the result of innovation, yes—but also of a system designed to protect profits. Knowing the difference between what’s necessary and what’s just branded gives you real power over your health and your wallet.
Below, you’ll find real stories and facts about how brand-name drugs affect patients, how generics compete, and when the system works—or fails—you.