When someone gets a new kidney, liver, or heart, the body doesn’t always recognize it as a gift-it sees it as an invader. That’s where azathioprine comes in. This older but still widely used drug helps stop the immune system from attacking the transplanted organ. It’s not flashy, it doesn’t make headlines, but for decades, it’s been a quiet hero in transplant care.
What Azathioprine Actually Does
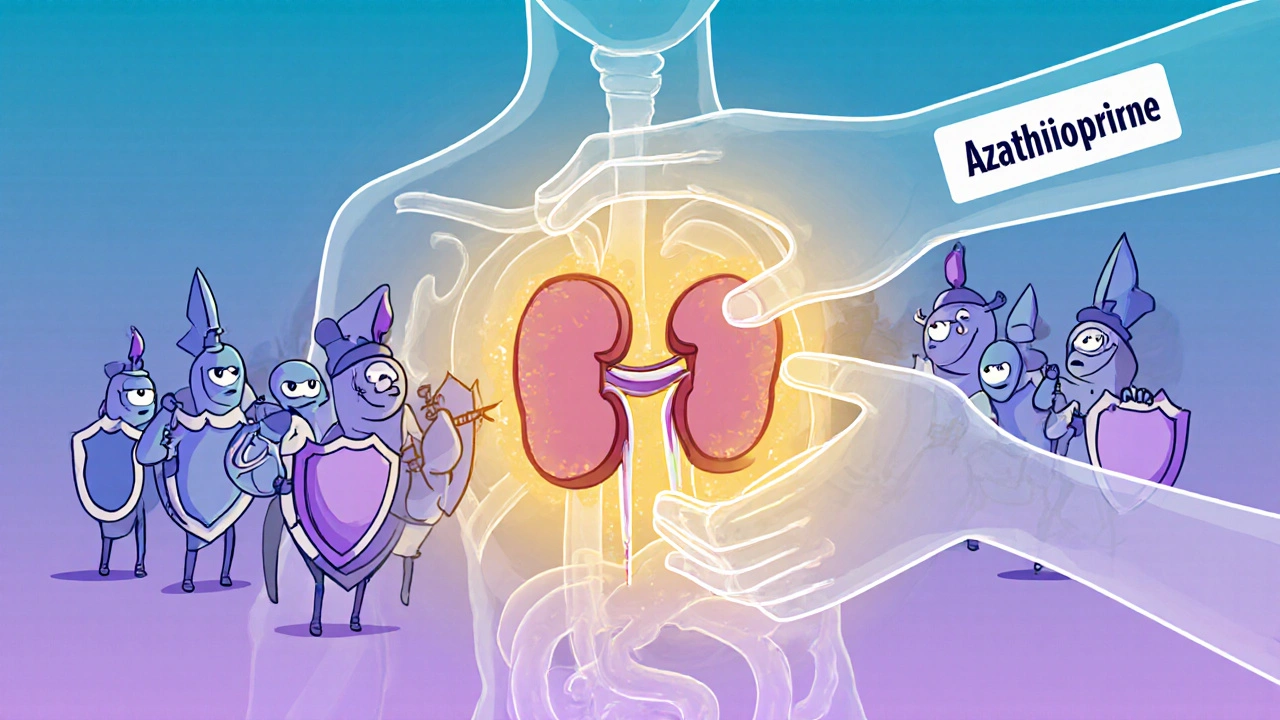
Azathioprine is an immunosuppressant. That means it slows down your immune system’s ability to fight off threats. After a transplant, your immune cells-especially T-cells-start hunting down the new organ like it’s a virus. Azathioprine interferes with the DNA-building process in these cells, so they can’t multiply fast enough to launch a full-scale attack.
It’s not a cure. It doesn’t make the organ feel like part of you. But it gives your body time to adjust. Most transplant patients take azathioprine for months or years, often alongside other drugs like corticosteroids or tacrolimus. The goal isn’t to shut down immunity completely-just to dial it back enough to let the transplant survive.
Why Doctors Still Prescribe It
You might wonder: if there are newer drugs like mycophenolate or sirolimus, why still use azathioprine? The answer is simple-it works, it’s cheap, and it’s well-understood.
Studies from the 1980s showed azathioprine improved one-year kidney transplant survival from about 50% to over 80%. Today, even with newer options, it’s still used in about 15-20% of kidney transplant cases in the U.S., especially in low-income settings or when cost is a barrier. In countries without universal healthcare, azathioprine can cost less than $50 a month, while newer drugs may run over $2,000.
It’s also used in pediatric transplants. Children’s immune systems are more active, so doctors sometimes start with azathioprine because its effects are easier to monitor and adjust over time.
How It’s Taken and How It Works in the Body
Azathioprine comes in tablet form, usually taken once or twice a day. The dose isn’t one-size-fits-all-it’s based on your weight, liver function, and how your body breaks the drug down. Some people have a genetic variation in the TPMT enzyme that metabolizes azathioprine. If you’re a slow metabolizer, even a standard dose can cause dangerous drops in white blood cells.
That’s why doctors often test for TPMT status before starting treatment. If you’re at risk, they’ll lower the dose or pick a different drug. This kind of personalized approach is one reason azathioprine has survived into the age of precision medicine.
The drug turns into 6-mercaptopurine inside your body, which then gets absorbed by immune cells. It doesn’t work right away. You might not feel any difference for weeks. That’s normal. It’s not a painkiller or a sleep aid-it’s a slow, steady hand on the brakes of your immune system.
Side Effects You Need to Watch For
Like all immunosuppressants, azathioprine comes with risks. The biggest one? Infections. With your immune system turned down, common viruses like colds or flu can become serious. You’re also at higher risk for skin cancer and lymphoma, especially if you’ve been on it for more than five years.
Other common side effects include nausea, vomiting, loss of appetite, and fatigue. These often get better after the first few weeks. But if you develop a fever, sore throat, or unexplained bruising, call your doctor. Those could be signs of low white blood cell counts.
Blood tests are required every 2-4 weeks at first, then monthly once your dose is stable. Monitoring your complete blood count and liver enzymes isn’t optional-it’s life-saving. Many transplant centers now use automated alerts to flag abnormal results before patients even notice symptoms.
Who Shouldn’t Take Azathioprine
It’s not for everyone. People with severe liver disease, active infections like tuberculosis, or a history of certain cancers usually avoid it. Pregnant women can take it under close supervision-it’s been used safely in transplant patients during pregnancy-but it’s not first-line for women planning to conceive soon.
If you’ve had pancreatitis before, azathioprine might trigger it again. And if you’re allergic to allopurinol, you can’t take azathioprine unless your dose is cut by 75%-because the two drugs interact in a way that can cause severe toxicity.
Some patients are switched off azathioprine because of side effects. Others stay on it for life. It depends on how well the transplant holds up, how your body handles the drug, and whether newer alternatives offer a clear benefit.
How It Compares to Other Immunosuppressants
| Drug | How It Works | Typical Dose | Cost (Monthly) | Key Risks |
|---|---|---|---|---|
| Azathioprine | Blocks DNA synthesis in immune cells | 1-3 mg/kg/day | $20-$60 | Infections, low blood counts, cancer risk |
| Mycophenolate | Stops white blood cell production | 720-1,440 mg twice daily | $1,500-$2,500 | Diarrhea, nausea, birth defects |
| Tacrolimus | Inhibits T-cell activation | 0.05-0.3 mg/kg/day | $800-$1,200 | Kidney damage, tremors, high blood sugar |
| Cyclosporine | Blocks T-cell signaling | 2-5 mg/kg/day | $300-$700 | Gum overgrowth, high blood pressure, kidney toxicity |
Each drug has trade-offs. Mycophenolate is more effective at preventing rejection but causes more stomach issues. Tacrolimus is stronger but harder on the kidneys. Azathioprine sits in the middle-less potent than newer drugs, but easier on the wallet and gentler on the gut for many people.
Real-Life Scenarios: Who Benefits Most
Take Maria, 52, who got a kidney transplant in 2020. She’s on Medicare but has a high deductible. Her doctor chose azathioprine because it fit her budget and her liver function was normal. She’s been on it for five years with no rejection episodes.
Compare that to Jamal, 28, who got a liver transplant and has a private insurance plan. His team switched him to mycophenolate because his immune system was highly reactive. He had mild nausea at first, but his rejection risk dropped from 30% to under 5% in the first year.
There’s no universal best drug. The right choice depends on your age, health, finances, and how your body responds. Azathioprine isn’t the most powerful tool in the box-but for many, it’s the right one.
What Happens If You Stop Taking It
Never stop azathioprine without talking to your transplant team. Stopping suddenly can trigger acute rejection-even if you’ve been stable for years. Rejection can happen within days. Symptoms include fever, swelling near the transplant site, fatigue, and sudden weight gain.
If rejection does occur, you’ll likely need high-dose steroids or even hospitalization. In some cases, the organ can be saved. In others, you might need another transplant. That’s why adherence is non-negotiable. Many clinics use pill dispensers with alarms or mobile apps to help patients stay on track.
What’s Next for Azathioprine?
It’s not being replaced-it’s being refined. Researchers are now studying how to use lower doses of azathioprine combined with newer biologics to reduce long-term cancer risk. Some trials are testing genetic screening to predict who’ll respond best, so patients aren’t stuck on a drug that doesn’t suit them.
For now, azathioprine remains a cornerstone. It’s not glamorous. But in the quiet, daily work of keeping a transplanted organ alive, it’s still doing its job.
Can azathioprine cure organ rejection?
No, azathioprine doesn’t cure rejection-it prevents it. It works by suppressing the immune system so it doesn’t attack the transplanted organ. If rejection has already started, stronger treatments like intravenous steroids or antibody therapy are needed.
How long do people take azathioprine after a transplant?
Most patients take azathioprine for life, especially if they’re not on newer drugs. Some may switch to other immunosuppressants after a few years, depending on side effects or rejection risk. Others stay on it for decades with no issues.
Is azathioprine safe during pregnancy?
Yes, it’s considered one of the safer immunosuppressants during pregnancy, especially compared to drugs like mycophenolate, which can cause birth defects. But it must be closely monitored. Transplant patients who become pregnant need frequent blood tests and high-risk obstetric care.
Does azathioprine cause weight gain?
Not directly. But because it’s often used with corticosteroids like prednisone-which do cause weight gain-many patients gain weight while on combination therapy. Diet and exercise help, but the main culprit is usually the steroid, not azathioprine.
Can I drink alcohol while taking azathioprine?
Moderate alcohol is usually okay, but heavy drinking is dangerous. Both azathioprine and alcohol stress the liver. Combined, they increase the risk of liver damage. Most doctors recommend limiting alcohol to one drink per day or avoiding it entirely, especially if your liver enzymes are already elevated.
What happens if I miss a dose?
If you miss one dose, take it as soon as you remember-if it’s not close to your next scheduled dose. Never double up. Missing doses increases rejection risk. If you miss more than two doses in a row, contact your transplant team immediately.




Joe Puleo
October 28, 2025 AT 05:48Azathioprine might not be sexy, but it's kept thousands of people alive when they couldn't afford the fancy stuff. I've seen patients on it for 15+ years with zero rejection. It's not perfect, but it's reliable.
Keith Bloom
October 29, 2025 AT 02:56lol at people acting like azathioprine is some miracle drug. yeah it's cheap but it's basically chemical soup that turns your blood into swamp water. i know a guy who got lymphoma after 7 years on it. they just don't tell you that part.
Ben Jackson
October 29, 2025 AT 21:47For those of you new to immunosuppression, azathioprine is the OG. It's not about being the strongest-it's about being sustainable. The TPMT testing alone? That's precision medicine before it was cool. Docs still use it for a reason: it's got decades of real-world data behind it.
Bhanu pratap
October 31, 2025 AT 10:34God bless azathioprine! In my village in India, this drug is a miracle. A man from my town got a kidney transplant and now he runs a small shop. He takes this pill every day like a prayer. No fancy names, no expensive bottles-just this little tablet keeping him alive. Thank you, science!
Meredith Poley
November 2, 2025 AT 06:04Wow, a drug that causes cancer but is cheaper than your monthly Netflix subscription. Truly the pinnacle of American healthcare innovation. Let me guess-the pharmaceutical companies love it because it’s profitable AND deadly? Brilliant.
Mathias Matengu Mabuta
November 3, 2025 AT 12:43It is imperative to note, however, that the statistical efficacy of azathioprine in preventing acute rejection-while historically significant-is not statistically superior to newer agents in multivariate analyses when adjusted for comorbidities, age, and genetic markers. The persistence of its use is, therefore, a function of institutional inertia and economic constraint, not clinical superiority.
Ikenga Uzoamaka
November 3, 2025 AT 19:51WHY DO THEY STILL USE THIS TOXIC DRUG?!?! I read the side effects and my heart stopped!! Cancer?! Low white cells?! You're telling me people are on this for DECADES?!?! My cousin died from this!!! Someone needs to stop this!!
Lee Lee
November 5, 2025 AT 06:31Think about it… who really benefits from azathioprine? Not the patient. Not even the doctor. It’s the insurance companies. The government. The hospital systems. They don’t care if you live 20 years with a 3% cancer risk-they care that you’re not costing $2,000 a month. This isn’t medicine. It’s a cost-cutting algorithm dressed in a lab coat.
John Greenfield
November 6, 2025 AT 01:41Ben Jackson is wrong. Azathioprine isn't 'sustainable.' It's a relic. My cousin’s transplant team switched her to mycophenolate after one year because her TPMT levels were borderline. She’s been rejection-free for five years. Azathioprine is a gamble with your life. Why risk it when better options exist?
Joe Puleo
November 7, 2025 AT 09:05John, I get your point. But not everyone has access to those 'better options.' My sister’s on Medicare with a $10k deductible-she can’t afford $2k/month. Azathioprine isn’t ideal, but it’s her only shot. The real problem isn’t the drug-it’s a system that forces people to choose between survival and bankruptcy.