Every pill, injection, or capsule you take has passed through a final, non-negotiable checkpoint before reaching your hands: batch release testing. This isn’t just paperwork or a formality. It’s the last line of defense between a potentially dangerous drug and a patient who trusts it will work safely. In 2025, with global supply chains stretched thin and complex biologics replacing simple tablets, this process has never been more critical.
What Exactly Happens During Batch Release Testing?
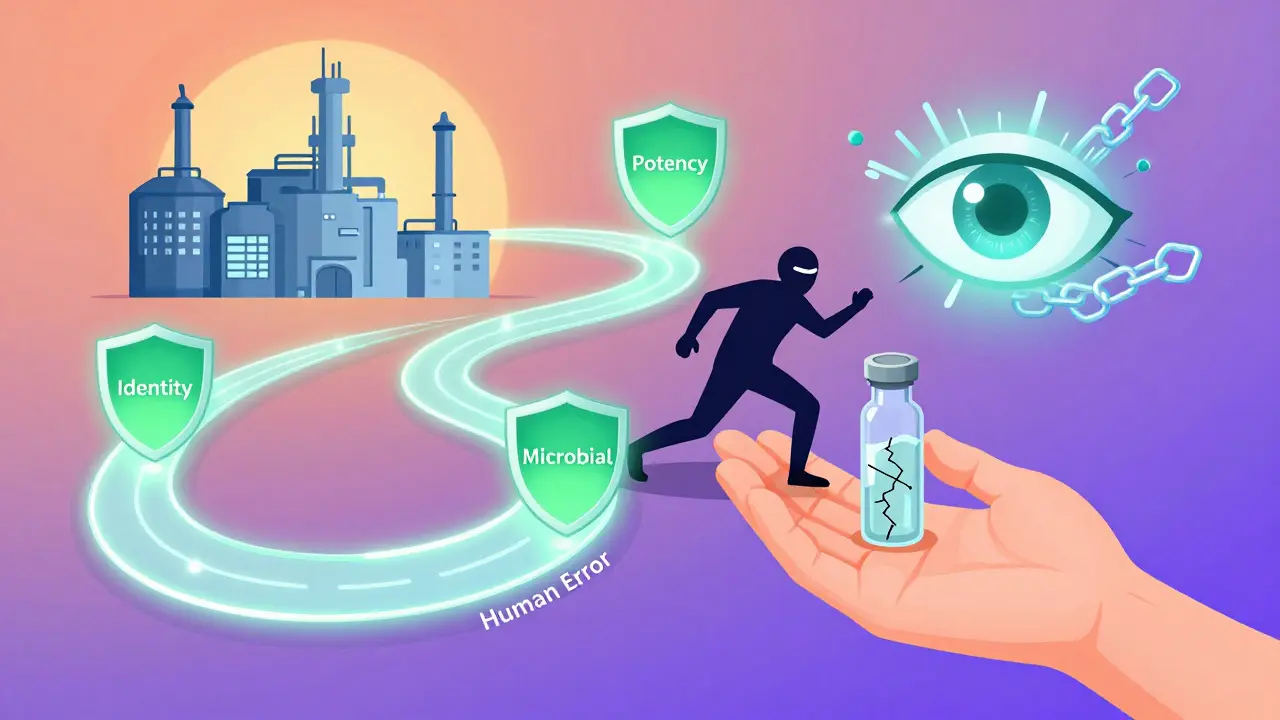
Imagine a factory produces 10,000 bottles of a blood pressure medication. Each bottle looks identical. But inside, one might have too little active ingredient. Another might have a hidden contaminant. Batch release testing finds those outliers before they leave the facility. It starts with physical and chemical tests. Each batch is sampled and analyzed using validated methods-often from the USP (United States Pharmacopeia) or EP (European Pharmacopoeia). These aren’t random checks. They’re precise, repeatable procedures that measure:- Identity: Is the drug actually what it claims to be? HPLC or FTIR machines confirm the molecular fingerprint matches the approved formula.
- Assay/Potency: Does it contain the right amount of active ingredient? Acceptable range is usually 90-110% of the labeled dose. Anything outside that gets rejected.
- Impurities: Are there unwanted chemicals? ICH Q3 guidelines set strict limits-like 0.10% for unknown impurities in new drugs. Even trace amounts can cause allergic reactions or long-term damage.
- Microbial limits: For non-sterile products, no more than 100 colony-forming units per gram. Sterile products? Zero tolerance. Every vial is tested for endotoxins using USP <85> methods.
- Dissolution: Will the pill break down properly in your body? Generic drugs must match the brand-name version’s dissolution profile (f2 similarity factor ≥50).
- Particulate matter: For injectables, any visible or microscopic particles are flagged. Small volume parenterals can’t have more than 6,000 particles ≥10μm per milliliter.
Why Is This Step So Strict?
Because one bad batch can cost lives-and millions. In 2023, the FDA reported that the average pharmaceutical recall costs $10.7 million. That’s not just lost product. It’s legal fees, lost trust, regulatory fines, and sometimes, lawsuits. A single batch of a monoclonal antibody released with subpotent levels in 2023 led to a $9.2 million recall and an 18-month import ban. That’s not an outlier. It’s a warning. Dr. Jane Smith, former director of the FDA’s Center for Drug Evaluation and Research, said in 2023 that batch release testing blocked about 1,200 unsafe batches from reaching U.S. patients that year alone. That’s 1,200 chances for harm that were stopped before they ever left the lab. The stakes are even higher for biologics-drugs made from living cells. These are fragile. A small change in temperature, pH, or mixing time can alter their structure. Testing for these products is more complex, takes longer (21-35 days), and costs more. Yet, they’re the fastest-growing segment of the market.Who Signs Off on a Batch?
In the U.S., a designated quality unit representative reviews all test results, manufacturing records, and deviations before approving release. But in Europe, it’s different. There, a Qualified Person (QP) must personally certify each batch. A QP isn’t just any lab manager. They need at least five years of pharmaceutical experience, specific GMP training, and formal certification. Europe currently has a 32% shortage of QPs, according to the EMA’s 2024 report. That means bottlenecks. Delays. Backlogs. Senior QP Maria Gonzalez from the Biophorum Operations Group shared that reviewing documentation for a single complex biologic batch can take 40-60 hours. And that’s just the paperwork. The actual testing? That’s done by a team. The review? That’s on one person’s shoulders.
How Long Does It Take?
Timing varies by product type:- Small molecule generics: 7-10 days
- Complex generics (like inhalers or injectables): 14-21 days
- Biologics (monoclonal antibodies, vaccines): 21-35 days
What Goes Wrong?
Despite all the rules, things still fail. According to the Parenteral Drug Association’s 2024 report, 83% of batch failures happen in three areas:- Dissolution (32%)
- Impurity profiles (28%)
- Microbial contamination (23%)
Technology Is Changing the Game
Many companies are adopting LIMS (Laboratory Information Management Systems) to automate data collection and review. A 2024 AAPS survey found that 65% of users saw a 22% speed-up in batch release cycles. Thermo Fisher’s SampleManager was named in 41% of those success stories. AI is next. Companies using AI-driven analytics for batch decisions report 34% fewer failures. But regulators are cautious. The EMA’s 2024 pilot showed AI matched traditional methods 78% of the time. The FDA wants 99.9% confidence before fully accepting it. New guidelines like ICH Q14 (effective Nov 2024) allow more flexible, risk-based testing. For well-established products, you might test fewer parameters. That saves time and money without compromising safety.What’s Next?
By 2028, 45% of batch release decisions could involve AI or real-time monitoring, according to McKinsey. But don’t expect traditional testing to disappear. Deloitte’s 2025 outlook says 60% of facilities using advanced manufacturing may reduce discrete batch testing by 2030. But legacy products? They’ll still need full testing through 2035. The FDA is also pushing for blockchain-based traceability by 2028. Every step-from raw material to patient-would be digitally recorded. That’s not just for compliance. It’s for speed. If a problem pops up, you can trace it to one machine, one shift, one batch in minutes, not weeks.Bottom Line
Batch release testing isn’t glamorous. It’s tedious. It’s expensive. It’s slow. But it’s essential. Every time you take a pill, you’re relying on hundreds of hours of lab work, dozens of checks, and the judgment of trained professionals who refuse to let a single unsafe batch slip through. The system isn’t perfect. There are delays. Shortages. Human errors. But the data shows it works. It prevents disasters. It saves lives. And until we can guarantee every drug is perfect from start to finish, this final check will remain the most important step in pharmaceutical manufacturing.Is batch release testing required by law?
Yes. In the U.S., it’s mandated under 21 CFR 211.165. In the EU, it’s required by Directive 2003/94/EC. No batch can legally be sold without passing these tests and receiving formal certification. Skipping it is a violation of Good Manufacturing Practice (GMP) and can lead to shutdowns, recalls, or criminal charges.
How often is batch release testing done?
Every single batch must be tested. There are no exceptions under traditional manufacturing rules. Even if you’ve made 100 identical batches before, the 101st still goes through the full testing process. The only exception is for facilities approved for continuous manufacturing under FDA’s 2023 guidance, where real-time monitoring may replace some lab tests-but only after rigorous validation.
What happens if a batch fails release testing?
The batch is quarantined and rejected. It cannot be sold or distributed. The company must investigate why it failed-was it a manufacturing error? A contaminated raw material? A faulty test? Then they must document the root cause, take corrective actions, and may need to reprocess or destroy the batch. If the issue is systemic, regulators may inspect the facility and impose restrictions.
Can batch release testing be automated?
Parts of it already are. Sample handling, data collection, and report generation are often automated through LIMS. AI is being used to predict outcomes based on process data. But final certification still requires human review. Regulators won’t let a machine sign off on patient safety. The Qualified Person or quality unit representative must still verify everything before release.
Why does it take so long to release a batch?
Because every test needs time. Dissolution tests run for hours. Microbial tests require 5-7 days of incubation. Stability data for new products can take months. Even with automation, you can’t rush biology. Plus, documentation must be reviewed by two people. For biologics, it’s even longer because the molecules are more complex and sensitive. Speed isn’t the goal-safety is.
Are generic drugs tested the same way as brand-name drugs?
Yes. Generic drugs must meet the same identity, strength, purity, and dissolution standards as the brand-name version. The FDA requires bioequivalence studies and full batch release testing. The only difference is that generics don’t need to repeat clinical trials-they just need to prove they behave the same way in the body. That’s why dissolution testing is so critical for generics.
What’s the difference between batch release testing and stability testing?
Batch release testing confirms a batch meets specs at the time of release. Stability testing checks how the product changes over time under different conditions-heat, humidity, light. Stability data ensures the drug remains safe and effective until its expiration date. Both are required, but they serve different purposes. You can’t release a batch without release testing. You can’t keep selling it without stability data.
Do over-the-counter (OTC) drugs go through batch release testing?
Yes. Even OTC drugs like ibuprofen or antacids must meet FDA or equivalent regulatory standards. While the testing might be less complex than for prescription biologics, every batch still undergoes identity, assay, and microbial testing. The same regulations apply-it’s just that the consequences of failure are often less severe because these drugs have wider safety margins.




Diane Hendriks
January 16, 2026 AT 13:58Every time I see someone in Congress complain about drug prices, I want to scream. You want cheap pills? Fine. But then don’t cry when the next batch kills someone because someone cut corners. This testing isn’t bureaucracy-it’s the only thing standing between you and a corpse wrapped in a prescription label. The US leads the world in this. Don’t let global supply chains or ‘efficiency’ erode that. We don’t outsource safety. Ever.
And yes, I know the QP shortage is real. Fix it. Hire more. Pay them more. Or stop pretending we care about patient safety.
Stop romanticizing ‘speed.’ Speed kills. Especially in pharma.
Frank Geurts
January 17, 2026 AT 18:49It is, indeed, a matter of profound significance-nay, of existential gravity-that each and every batch of pharmaceutical product, whether it be a humble ibuprofen tablet or a multi-million-dollar monoclonal antibody, undergoes exhaustive, meticulously validated, and legally codified release testing. The United States Pharmacopeia, the European Pharmacopoeia, and the International Council for Harmonisation have, through decades of painstaking collaboration, established a framework of scientific rigor that, while imperfect, remains the most robust safeguard of public health ever devised by human ingenuity.
It is not merely a regulatory checkbox. It is, in fact, a covenant-a solemn, unbroken promise between the laboratory and the patient. To diminish it, even slightly, is to betray the very foundation of medical ethics. And yet, we see, in the shadows of cost-cutting and automation, the faintest whispers of erosion. We must not listen to them.
Nat Young
January 18, 2026 AT 12:53Let’s be real: 83% of failures are from people? Shocking. No, really. Who knew? Oh wait-everyone in pharma has known this since 2010. The real problem? Companies spend $20M on LIMS but $500K on training. AI’s gonna fix it? Lol. The same AI that misclassified a pill as a rock in the training data? Great.
And ‘predictive release’? Sounds like a corporate buzzword for ‘we’re tired of waiting 3 weeks so we’re gonna gamble with your life.’
Also, why is the FDA letting biologics skip testing if they ‘look good’? Because they’re scared of the backlog. Not because it’s safe. Don’t believe the hype.
Niki Van den Bossche
January 20, 2026 AT 00:59There’s something haunting about this whole system, isn’t there? The quiet, sterile labs, the hum of HPLC machines, the fluorescent glow over chromatograms like sacred scrolls…
We’ve turned human life into a statistical probability. A 90–110% potency window? A 0.10% impurity threshold? We’ve reduced the miracle of healing to a spreadsheet. And yet-we trust it. We hand our children’s fevers, our parents’ heart conditions, our own existential dread over a diagnosis… to a machine reading a peak at 12.34 minutes.
It’s beautiful. And terrifying. We don’t just rely on science-we deify it. And when it fails? We’re not angry at the system. We’re angry at ourselves-for believing it could ever be perfect.
Maybe the real drug isn’t in the bottle. Maybe it’s in the silence between the test results. The breath held. The finger hovering over ‘Approve.’
Iona Jane
January 21, 2026 AT 10:11They’re lying. All of them. The FDA, the EMA, the ‘qualified persons’-they’re all in on it. Batch testing? A myth. The real tests are done in private labs owned by Big Pharma. The ‘failed’ batches? They get repackaged. Re-labeled. Sold as generics overseas. That $9.2M recall? A PR stunt. The actual bad stuff? Still in circulation. You think they’d let a batch with endotoxins out? No. They just retest it after bribing the lab tech. The system’s rigged. Blockchain? That’s just a new way to hide the fraud.
And don’t get me started on AI. They’re training it on fake data. They’ve been doing this since the 90s. You think your insulin is safe? It’s not. It’s been altered. I know because I’ve seen the documents. They’re coming for your meds next. Prepare.
ellen adamina
January 23, 2026 AT 09:09I’ve worked in a hospital pharmacy for 12 years. I’ve seen the paperwork. I’ve held the vials. I’ve watched the QC team stay until 2 a.m. because a dissolution profile was off by 0.7%.
They don’t do it for the money. They do it because they know someone’s kid is going to take this tomorrow. Someone’s grandma. Someone’s husband.
I don’t care how slow it is. I don’t care how expensive. I just want to know that the person who signed off on it cared as much as I do.
Thank you to every lab tech, QP, and auditor out there. You’re the real heroes.
Sohan Jindal
January 24, 2026 AT 03:42Why are we letting foreign labs test our drugs? China, India, they don’t care about our kids. This whole system is a joke. We’re trusting our health to people who speak a different language and work for pennies. And now they want to replace humans with AI? Are you kidding me? We need American QPs. American labs. American testing. No more outsourcing. No more shortcuts. This isn’t about cost. It’s about loyalty. And we’ve lost it.
Fix it. Or I’m taking my business to Canada.
Mike Berrange
January 25, 2026 AT 09:43Everyone’s talking about AI and blockchain like they’re magic bullets. But the real problem? The people who write the SOPs. The ones who don’t read them. The ones who sign off without looking. The ones who say ‘it’s close enough.’
Automation doesn’t fix human laziness. It just makes it faster.
And yes, I’ve seen the Form 483s. I’ve seen the ‘corrective actions’ that were just reworded versions of the same mistake. We’re not fixing the system. We’re just making it look better on paper.
Until we stop rewarding speed over accuracy, nothing changes. And we all know it.